By B. Monrabal. Polymer Char, Valencia, Spain. Wallace W. Yau. Polymer Char Scientific Consultant, Texas, USA.
A review of the technological advances in High-Temperature GPC/SEC instrumentation through time.
Introduction
The molar mass distribution is, in effective terms, the microstructure definition of a polydisperse homopolymer resin of known chemistry. Before gel permeation chromatography (GPC) or size exclusion chromatography (SEC) was developed, chemists had to accept an incomplete characterization of their polymer materials measuring only molar mass averages by osmometry or static light scattering. Even so, these techniques demanded considerable effort when dealing with synthetic polymers that were not soluble at room temperature.
All that came to a drastic end when, in the 1960s, the development of GPC by Moore opened up the possibility of measuring the full molar mass distribution in a relatively short time 1. It was found that molecules of different size were separated in a column packed with porous particles; the smaller the molar mass, the more pores they would permeate and the longer they would take to elute from the column. Furthermore, the timing was perfect to combine Moore’s newly developed GPC columns with Waters’ differential refractive index (RI) detector.2 With that, a new instrument known as the GPC 100 was born. It was larger than a refrigerator and weighed five times as much.
This first GPC instrument incorporated a pump, an injection valve, a column oven and a RI detector. Quite some engineering ingenuity was required in those days to have a constant flow pump that was particularly required in GPC, where a small change in flow could cause large molar mass errors, and to have a stable RI detector at high temperature (with RI being known to have strong temperature dependency). It is interesting to notice that the very first GPC was targeted at high-temperature applications. This certainly showed the importance that the knowledge of the molar mass distribution of polyolefins had in the design of new resins. This leads us on to discuss polyolefins and high-temperature GPC in terms of engineering advances.
Technical Milestones
Autosamplers: In the 1980s the most significant engineering achievement was automation. This was important as the GPC analysis time is relatively short and the demand for GPC analysis was growing exponentially. Instruments with integrated autosampler and even polymer solutions filtration (to remove gels that could damage the columns) appeared on the market. However, these automated features did not always work well and most users still had to do these steps in an external fume hood and transfer the hot sample solution into the GPC analytical vials to be finally injected; this was obviously of concern at the expense of time and the safety issue of handling hot solvent.
Viscometer and light-scattering detectors: A significant breakthrough was the development and integration of viscometer and multi-angle light scattering detectors in GPC instruments,3,4which provide extended information on branching and more precise molar mass distribution.
A GPC system equipped with an online light-scattering (LS) detector and an online viscometer in addition to an RI concentration detector was commonly known as the triple detector GPC (TD-GPC or 3D-GPC). In such system, absolute molar mass distribution and absolute intrinsic viscosity distribution could be determined across the GPC elution curve in a single experiment. The combination of these results provided a powerful technique of measuring polymer long chain branching (LCB) while the molar mass distribution obtained by conventional calibration in the same experiment provided the information on the polymer chain backbone distribution. An example of the TD-GPC result plotted versus the elution volume can be seen in Figure 1.
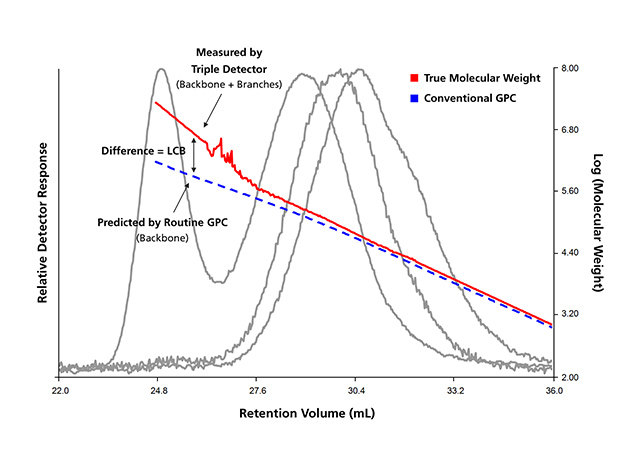
Figure 1: Triple-detector GPC results of a low density polyethylene (LDPE)
Column technology: Around the same time, column manufacturers were improving the column packing technology in terms of resolution while maintaining particle strength, blending different pore size particles to provide linear calibration, increasing temperature stability and improving efficiency (by using more uniform and smaller particle size). Later on, it was necessary to increase particle size again because very large molecules were found to suffer shear degradation when using small particle columns, especially for low-density polyethylene (LDPE) samples containing fragile LCB structures.5
Autosamplers with different heated zones: As equipment began to accommodate more sample vials, new autosamplers with two separate heated zones were designed to reduce sample degradation while waiting for injection. In polyolefin analysis, however, the analyst still had to perform sample preparation and filtration externally, and to go through a high temperature solution transfer from the filtration vial to the GPC analytical vial. The thermal degradation, which was more severe for polypropylene than polyethylene, is illustrated in a recent study and shown in Figure 2.6
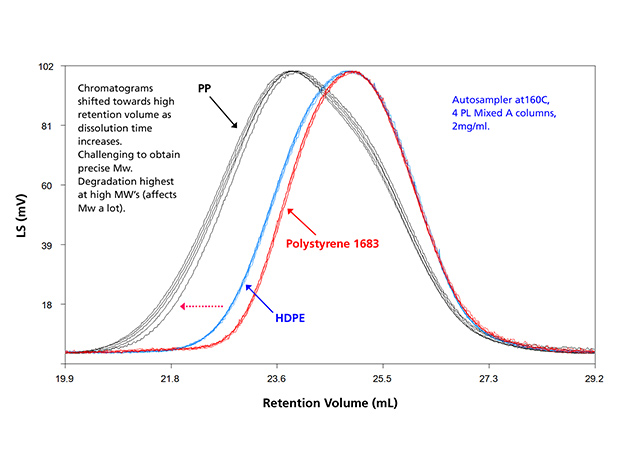
Figure 2: Thermal degradation of multiple injections from sample vials located from the start to end of the sample carousel. PP shows high variation due to thermal degradation.
New detectors: The refractive index detector has remained the most popular and universal detector in GPC. In the case of polyolefins analysis, infrared (IR) detection was shown to be more appropriate in the 1970s although IR technology was not yet fully developed.
Following the introduction of Fourier Transform IR (FTIR), infrared detection attached to GPC became interesting again in the 1990s for obtaining the composition molar mass dependence in polyolefins by measuring methyls per 1000 carbons with a flow-through cell. FTIR spectrometers demanded the use of mercury cadmium telluride (MCT) detectors for good sensitivity but needed to operate at liquid nitrogen temperatures. The use of chemometrics to improve signal-to-noise ratio has also been reported.7
In the 1990s, single, dual and multi‑angle light-scattering detectors with improved performance were consolidated. Light‑scattering detectors are now being used as standard in many GPC applications.8
In the 2000s new optoelectronic infrared detectors of high sensitivity were developed using interference filters at selective wavelengths.9 They soon became very popular for GPC analysis of polyolefins because of their higher sensitivity, short stabilization time and stability against temperature fluctuations. Infrared detectors are, however, restricted to applications where the solvent is transparent enough in the spectrum region for sample absorption to be detected. More recently a new filter type infrared detector with an MCT thermoelectrically cooled sensor has appeared avoiding the need for liquid nitrogen cooling.10 The IR detector for polyolefin analysis has the advantage of providing a cleaner detection of sample components in the low molar mass tail of the GPC elution curve. The presence of solvent impurities and air or water peaks often interferes with the RI detection in this low molar mass region. This difference is shown in Figure 3, where both IR and RI detectors are used in addition to LS and viscometry.11
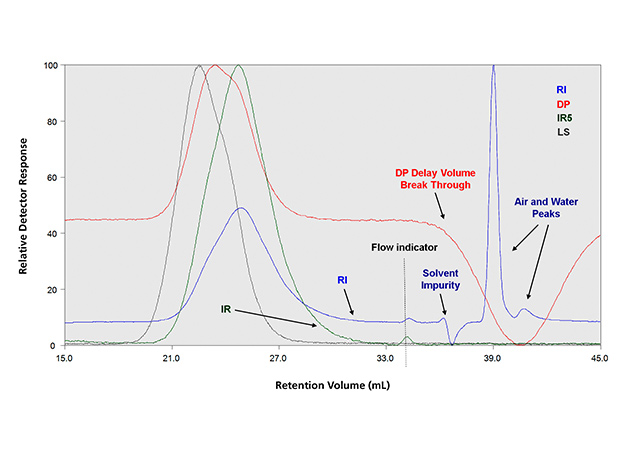
Figure 3: A typical detection result of a quadruple detector GPC experiment that shows (i) the difference between IR and RI in the low molar mass region and (ii) the viscometer delay volume breakthrough peak.
The analysis of 1476 LDPE resin using IR, viscometry and LS is presented in Figure 4, which shows the greatly improved TDGPC results over those back in the 1990s, because of the much improved IR and LS detectors. A successful viscometer design is implemented as well, avoiding the delay in volume breakthrough peak to achieve a reduced analysis time in the TD-GPC experiment.
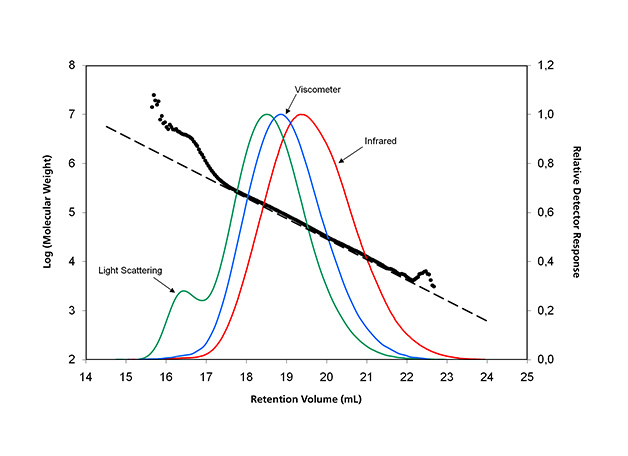
Figure 4: The triple-detector GPC results of a 1476 LDPE resin.
Infrared detection with multiple wavelength sensing elements measures concentration and composition simultaneously; this is of special interest for high-density polyethylene (HDPE) pipe resins, ethylene-propylene copolymers and ethylene vinyl acetate resins. This capability can be demonstrated in Figure 5 for three different classes of polyolefin applications: (a) a series of single-site catalyst copolymer resins, (b) the analysis of a pipe resin and (c) an ethylene-propylene copolymer. These co-monomer distribution results are as important as, and complementary to, the additional molar mass distribution information for the characterization of polyolefins.
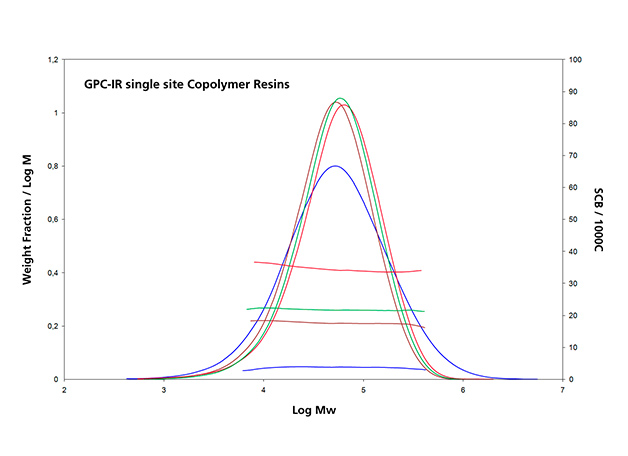
Figure 5.a: Composition analysis by an IR dual wavelength detector of single-site catalyst resins of different composition,
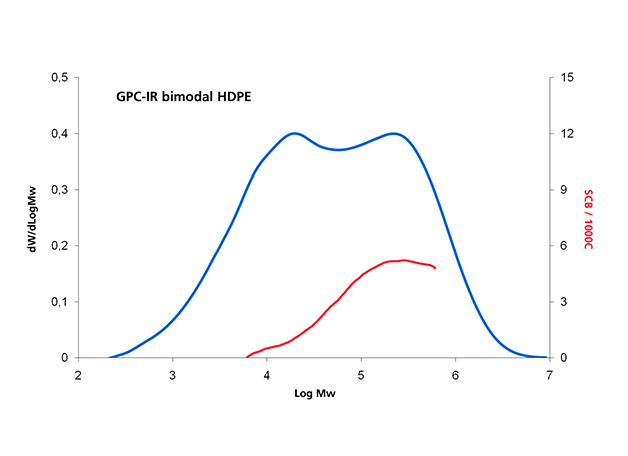
Figure 5.b: Composition analysis by an IR dual wavelength detector of HDPE pipe resin
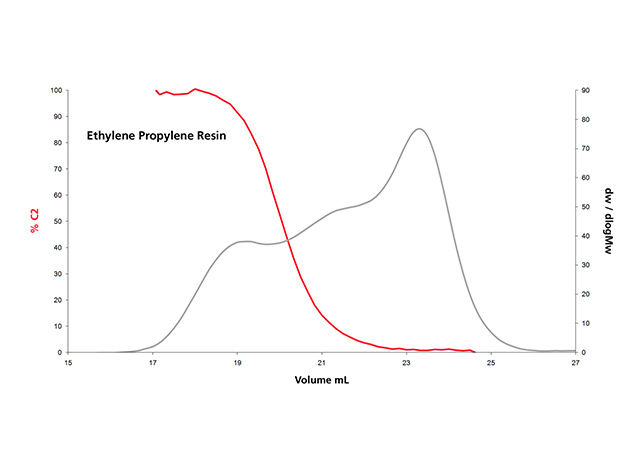
Figure 5.c: Composition analysis by an IR dual wavelength detector of of a complex polyethylene–polypropylene resin.
Most Recent Engineering Advances
Besides the new detectors, modern GPC instruments have to improve precision in the molar mass determination. Precision is highly dependent on the precision of the flow‑rate; a small flow‑rate variation of 1% may result in 20% error in Mw.12 A significant improvement in molar mass precision is obtained by the automation of adding flow markers to sample vials.
Another source of error can come from the sample overloading. GPC experiments should be run at high detector signals by using high sample concentrations but not high enough to cause sample overloading. Sample overloading tends to cause an underestimate of the molar mass results.13 The use of the most sensitive detectors is always preferred.
In the last few years new GPC instrumentation has been introduced with modular design that facilitates maintenance tasks. Sample vials are of higher volume, 10 to 20 mL, to reduce error by lack of sample homogeneity. The tedious and time-consuming vial transfer is not required anymore. Another important achievement is to have a column oven separated from the injection and detector oven compartment facilitating maintenance tasks without causing large heating and cooling stresses on GPC columns that can shorten the column life.
To prevent sample degradation, new designs incorporate the following:
• Vials purged with nitrogen, before filling with solvent to prevent polymer thermal degradation, which is particularly
important for polypropylene samples.
• Sample dissolution performed by a gentle shaking action to prevent shear degradation.
• Samples staying at high temperature for just the required time for dissolution to minimize thermal degradation.
Full automation in high-temperature GPC is most important for minimizing hot solvent handling, which nowadays is achieved by:
• Vials automatically filled with solvent by an internal syringe dispenser.
• A flow marker being injected automatically into the vial.
• Sample solutions automatically filtered in the process of filling the injection loop. The filter is backflushed and cleaned automatically after each injection.
All engineering advances in hardware occur in parallel with those of software control design, facilitating remote control diagnosis.
The software has been made flexible enough to allow users to implement personalized algorithms with advanced features of band broadening correction, traces review, and statistical quality control (SQC) chart for tracking key GPC parameters.
Conclusion
Significant improvements in high-temperature GPC operation have been achieved in the last few years. Improved detectors for better sensitivity, improved pumps and less fragile GPC columns are now standard.
Multiple detectors: Concentration, viscometry, light scattering, and composition detections are now being used in many GPC systems. The reliability and ease of maintenance of the instrument are of utmost importance for GPC users. These are essential requirements for working at high temperatures and very often with difficult solvents. Automating sample preparation with the least interaction from the analyst has been achieved, while at the same time taking care to prevent sample degradation.
References
1. J.C. Moore, J. Polym. Sci., A2, 835–843 (1964).
2. L.S. Ettre, LC•GC N. Am., 23(8), 752–761 (2005).
3. M.A. Haney, J. Appl. Polym. Sci., 30, 3037 (1985).
4. P.J. Wyatt, Anal. Chim. Acta, 272, 1 (1993).
5. A.W. deGroot and W. Hamre, J. Chromatogr.,648, 33–39 (1993).
6. R. Cong et al., ISPAC 2007 Proceedings (2007).
7. P. DesLauriers, D. Rohlfing and E. Hsieh, Polymer, 43, 159–170 (2002).
8. A.M. Striegel et al., Modern Size-Exclusion Liquid Chromatography, Wiley, New York 2009, Chapter 9 “Physical Detectors”.
9. A. Ortín and B. Monrabal, International GPC Symposium, Las Vegas 2000.
10. J. Montesinos et al., 1st ICPC Conference, Houston, Texas, USA, October 2006.
11. T. Huang et al., 3rd ICPC Conference, Shanghai, China, November 2010.
12. W.W. Yau, J.J. Kirkland and D.D. Bly, Modern Size-Exclusion Liquid Chromatography, Wiley, New York 1979, pp. 228–229.
13. ibid., p.242.