February 2014
By B. Monrabal, A. Ortín, P. Del Hierro. Polymer Char, Valencia, Spain.
Introduction
The analysis of the amorphous fraction in polypropylene resins is considered a fundamental task in the production of polypropylene; it provides a measurement of the small amounts of undesirable low tacticity and low molar mass fractions in the homopolymer resins, which have a significant influence on the polymer properties and processing. The presence of amorphous fraction is required for certain applications, as in the case of high-impact polypropylene copolymer resins. The incorporation of the amorphous material is usually done within the same production plant in a second stage of the polymerization process by adding ethylene to the continuous reaction, which results in the formation of the amorphous ethylene-propylene copolymer. The continuous measurement of the overall amount of this rubbery fraction, which can range between 0 and 30% of the total polymer, is essential for plant control in order to obtain the desired specifications and performance of the polymer resin. In addition to the measurement of the total rubber percentage (soluble fraction), the analysis of its molar mass and ethylene content are of major importance in this type of manufacturing process.
Historically, the amorphous or soluble content has been measured by solubility in xylene using manual wet chemistry methods, as described in the next section, which demands significant manpower, solvent volume, and analysis time. Later on, it was shown that the amorphous content in polypropylene can be measured by crystallization (1) and a method was prepared based on the Crystallization Analysis Fractionation (CRYSTAF) technique (2-4). A new approach has now been developed to analyze polypropylene samples, one at a time, in Quality Control plant laboratories. CRYSTEX QC consists on a fully automated system based on a proprietary Temperature Rising Elution Fractionation (TREF) column where a small aliquot of the homogeneous polymer solution is crystallized on a support under reproducible and well-controlled conditions. The amorphous fraction is measured with a highly sensitive filter-type Infrared (IR) detector delivering equivalent values to the xylene solubles measurement, obtained with outstanding precision.
In addition to measuring the amorphous or soluble fraction percentage, CRYSTEX QC quantifies the ethylene incorporation and intrinsic viscosity in the original sample and in both, the amorphous and crystalline fractions. Total analysis time per sample is approximately two hours.
Classic Gravimetric procedure
Natta and coworkers (5) were among the first to understand the importance of separating the polypropylene produced according to stereo regularity. In those years (1950s) fractionation was only accessible by extraction, using a Soxhlet apparatus with solvents of different boiling points. The atactic polymer was obtained by the extraction in boiling ether, the stereo block fraction corresponded to the material which was insoluble in boiling ether but soluble in boiling heptane, and the isotactic fraction was what remained insoluble in those solvents. Most importantly in these extraction procedures was the determination of the amorphous atactic fraction, although reproducibility was not as good as desired.
A few years later (1960s), a new approach was proposed by P.M. Kamath, L. Wild (6) and others, by fully dissolving the sample in a good solvent and allowing it to precipitate according to its crystallizability. Carrying out the test in this manner and establishing the equilibrium in the opposite direction provided a significant advantage over the extraction procedure:
The amorphous fraction did not have to migrate from the interior of the solid material to the surface, which could be influenced by the size of the particles and the molecular structure of the matrix.
The solvent selection was not so important in this procedure, and from a variety of solvents, xylene was chosen due to the ease of filtration of the crystallized polymer and ease of drying.
This procedure, which they named Fractional Crystallization, resulted in a significant improvement in reproducibility over the extraction method and it showed that separation was barely affected by molar mass or by the initial concentration in a rather broad range.
The Fractional Crystallization approach finally evolved into the current standard procedures (7-8)which can be summarized as follows:
“The polypropylene is dissolved in hot xylene, then cooled under controlled conditions down to 25ºC, which results in the precipitation of the insoluble fraction. The soluble matter remains in the xylene. The suspension is filtered and an aliquot of the solution is then evaporated, dried and the residue weighed.” (7-8)
A schematic analytical process is depicted in Figure 1. This procedure, which we will refer to as the classic gravimetric method,has been extensively used in the industry and has proven to be of great value to the manufacturing of polypropylene (PP). The analysis of the xylene soluble with this approach however, demands significant manpower, solvent consumption, and an analysis time of around five to six hours. Just as significant is the fact that the precision of the method suffers from the required controlled precipitation conditions as discussed in the ASTM and ISO methods.
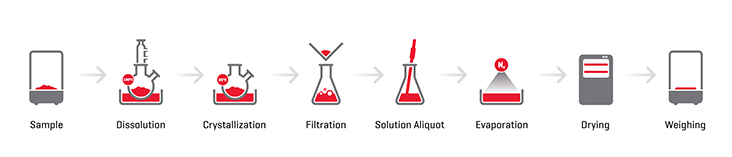
Figure 1. Schematic representation of the analytical steps demanded in the classic gravimetric method of xylene solubles determination.
In the last years, new methods have been developed to quantify the amorphous fraction attempting to overcome previous time-consuming procedures by automating the analytical process and, at the same time, provide extended characterization of the amorphous and crystalline phases.
Automated Crystallization methods
The classic gravimetric method, as discussed above, is based on a crystallization process where the crystalline matrix is segregated from the amorphous soluble fraction by cooling the solution according to a specified method with a fixed crystallization temperature and time. The selection of solvent did not appear to be of significant importance as the intention is to separate two different polymer phases quite apart in crystallinity as shown in Figure 2, where the soluble fraction corresponds to the distinctive peak in a PP resin characterized by TREF or CRYSTAF analysis.
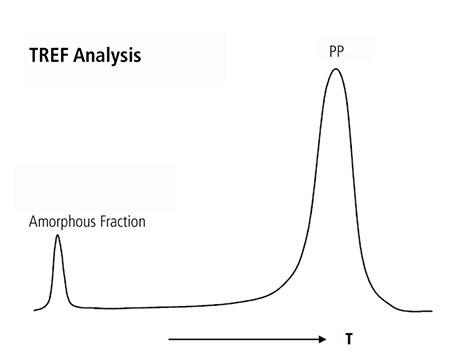
Figure 2. Chemical Composition Distribution of a polypropylene copolymer analyzed by TREF where the amorphous and crystalline fractions are well segregated.
In the early 2000s, it was shown that the amorphous content in polypropylene can be measured by an automated crystallization technique(1 ) and a method (CRYSTEX) was developed based on CRYSTAF (2-4) using a chlorinated solvent (di or tri-chlorobenzene) combined with the use of an IR detector. The correlation with the classic gravimetric method was excellent for a wide selection of PP resins as shown in Figure 3, while obtaining better reproducibility thanks to the full automation of CRYSTEX.
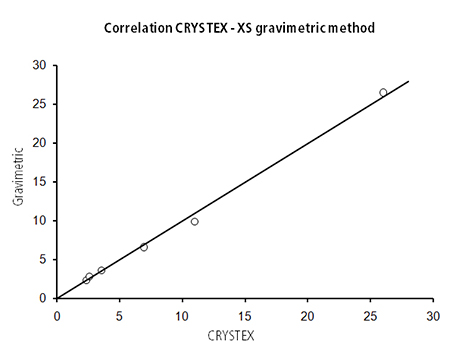
Figure 3. Correlation between the xylene solubles ISO/ASTM methods and the new crystallization approach.
An extension of the CRYSTEX technique for application in quality control laboratories of polypropylene plants has now been developed. The new analytical process, named CRYSTEX QC, is based on TREF, rather than the CRYSTAF approach, with the intention of speeding up the analysis of a single sample while maintaining the system’s same capability to measure intrinsic viscosity and ethylene incorporation. The new method analyzes one sample at a time and is capable to dissolve up to 4 g of sample, thus, minimizing error due to the poor homogeneity of powder samples.
The schematics of the system are presented in Figure 4. The only task done by the analyst consists in putting an approximate amount of sample into a bottle, placing the bottle in an oven, lowering a handle to pierce the bottle’s septum, and initiate the automated run. There is no need of weighing the sample since the IR detection of the full solution will provide accurate measurement of the amount of resin used, (which does not need to be in a dry form as residual water will not be detected by the IR). The IR detector is used to measure as well, the concentration of the amorphous and crystalline fractions and their ethylene incorporation. The crystallization in a TREF column, on an inert support, eliminates the filtration step, and the integration of a capillary viscometer provides an automated measurement of intrinsic viscosity of the whole sample and both amorphous and crystalline fractions.
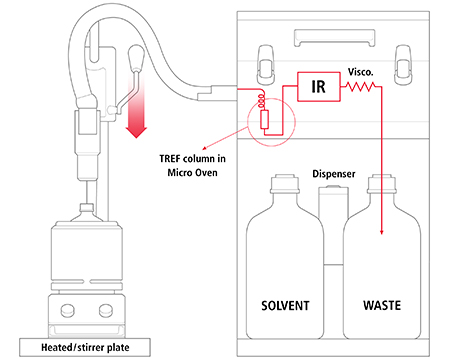
A major advantage of this approach is that the crystallization step is done with a small but representative aliquot of the solution and takes place in a packed column where the crystallization kinetics are fast and very well-controlled; thus, providing very reproducible results. The overall analytical process lasts two hours including automated solvent dispensing into the bottle to dissolve the sample, and column cleaning in order to be ready for the next sample analysis.
Once the powder or pelletized polymer is put into the bottle and the automated run is initiated, the analysis proceeds as follows:
- Filling the disposable bottle up to 100 or 200 mL with preheated solvent at 160ºC (depending on whether pellets or powder samples are analyzed) and initiate stirring. The deep vortex formed in the dissolution process prevents the polymer from sticking to the glass walls and speeds up dissolution.
- An aliquot of the solution is pumped through the TREF column at 160ºC towards the detectors to measure the whole sample concentration, ethylene incorporated and intrinsic viscosity (first eluted peak in Figure 5).
- A new aliquot of the solution is injected into the middle of the column at 160ºC, the flow is stopped and the column temperature is reduced rapidly down to controlled ambient temperature, staying there for an specified amount of time to precipitate the crystalline fraction; the column is then flushed to elute the amorphous soluble fraction peak (second peak in Figure 5). The ratio of this peak area by the one of the whole sample provides the soluble percentage in the sample. As the soluble material passes through the IR and Viscometer detectors, the ethylene content and intrinsic viscosity of this fraction are also measured.
- Temperature is raised rapidly up to 160ºC with stop flow to re-dissolve the crystalline fraction and after a pre-set time of a few minutes, this fraction is eluted through both IR and viscometer detectors.
- The column is washed and the instrument remains ready for a new sample analysis.
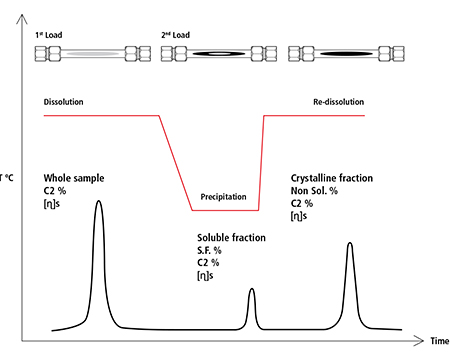
Figure 5. Elution of the whole sample and the PP fractions in the TREF column
Full analytical results are obtained in two hours, including not only the soluble fraction percentage, but also the ethylene content and intrinsic viscosity of whole sample, as well as of the soluble and crystalline fractions. Reproducibility in the soluble fraction percentage determination is remarkable; typically standard deviation (std) is lower than 0.1%, as seen in Table 1, much lower than indicated for the standard gravimetric methods.(7) In the same Table, whole sample ethylene and intrinsic viscosity are also reported, along with standard deviation (std) values. The precision achieved, also in those determinations, proves the reliability of this method.
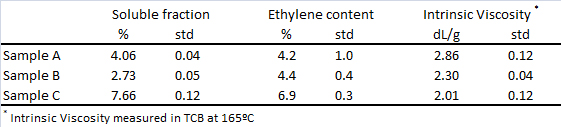
Table 1. Soluble fraction, total ethylene content and Intrinsic Viscosity for three different PP products. Data obtained from seven replicate analyses using 4 g of sample
Ethylene content and intrinsic viscosity data along with their std values for the analysis of soluble and crystalline fractions are shown in Table 2. It must be emphasized that no additional experimental effort is required, since the data is collected by the IR and viscometer detectors during the automated analysis. The precision in ethylene content for the crystalline fraction is as good as for the whole sample, while for the amorphous fraction the standard deviation will depend on the amount of soluble material. On the other hand, the intrinsic viscosity is determined with good precision for all the fractions.
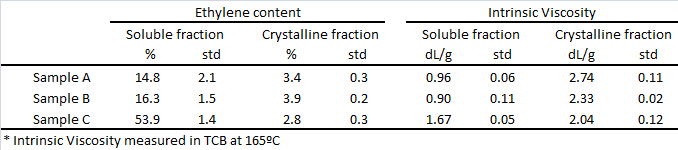
Table 2. Ethylene content and Intrinsic Viscosity in the amorphous/soluble and the crystalline fractions for three different PP products. Same analysis as in Table 1.
Conclusions
CRYSTEX QC is a new method to measure the soluble fraction in polypropylene resins has been developed based on a TREF crystallization approach. The analytical process is fully automated with no need of weighing, filtration or solvent handling.
This new method is especially interesting for quality control (product and process) in polypropylene plants. The total analysis time is two hours, and samples are analyzed one at a time in disposable bottles. Total volume consumption is as low as 180 mL for a 2-g sample analysis.
Analysis of 4 g of resin in the form of powder can be done for enhanced sample representativeness.
Besides the soluble fraction (equivalent to xylene solubles), CRYSTEX QC measures automatically and simultaneously, the ethylene content and intrinsic viscosity in both amorphous and crystalline fractions, and in the whole sample.
The possibility to measure a sample in two hours allows a fast response in process control during product grade changes reducing off grade production.
The new system can be used with other polyolefin type resins as well.
References
- Monrabal , Encyclopedia of Analytical Chemistry, John Wiley & Sons, 2000 pages 8074-8094.
- Romero, B. Monrabal, A. Ortín, Pittcon 2001 U.S.A.
- A. Ortín, B. Monrabal, M. D. Romero, LCGC Europe Vol 19 Issue Suppl., March 2006, 32.
- Ortín, L. Romero, B. Monrabal. Polyolefin Characterization (ICPC) November 7 – 10, Shanghai, China 2010
- Natta et al. Chim. e Ind. (Milano) 39, 275, 1957
- M. Kamath and L. Wild, Polymer Engineering & Science, 6, Issue 3, 213–216, 1966
- ASTM standard D5492-10, ASTM
- ISO standard 6427, 1992, ISO standard 16152, 2005