January 2017
By A. Fernández, N. Mayo, S. Gamiz, R. Chiva, B. Monrabal. Polymer Char, Valencia, Spain.
PREP C20 is powerful and sophisticated equipment designed to fractionate large amounts of polymer in a completely automatic way, which works as a pilot plant eliminating the need of manual handling of high volumes of hot solvent. A column-based instrument in which fractionation can take place according to the basis of Temperature Rising Elution Fractionation (TREF) technique, or according to molar mass using a solvent-non solvent gradient.
Introduction
Preparative fractionation in a column packed with an inert support is a long used technique for polyolefin characterization, as it facilitates the analysis of the fractions to obtain the bivariate distribution of a resin.
For decades, fractionation has been an important but challenging and tedious task because it requires manually handling of large volumes of toxic solvents at high temperature. Thus, the significant amount of time and effort demanded for the operation called for an automatic equipment that avoided solvent handling by the analyst.
In the 90’s, Polymer Char developed the instrument PREP mc2, an automated technique that was able to fractionate up to 2 grams of polyolefins according to their chemical composition (by temperature rising elution fractionation, TREF or by crystallization analysis fractionation, CRYSTAF) or according to their molar mass. Samples were placed into vessels and fractionation was performed automatically according to the selected method in less than 24 hours. Nevertheless, with this method, fractionation takes place with the absence of a support, and it has started to become evident that, for some polymers, when fractionating by TREF, a support for the solution to adhere to is critical for a good separation of the fractions, as is the case with multiple reactor-catalyst resins.
As a response, Polymer Char introduced the PREP C20, a column-based equipment, which – by adding a support for the solution to crystallize – improves the separation for each of the fractions, and therefore becomes more appropriate for those types of complex resins. Moreover, for a better understanding of the structure properties relationship, fractionation techniques are needed in order to separate polymers into fractions, which allow for their subsequent characterization. Often, some of the techniques employed to analyze the fractions require high amounts of polymer, which implies doing several fractionations of the parent sample to achieve the needed amount for the fractions.
In this way, PREP C20 has been designed to fractionate high amounts of polymer, up to 20 grams depending on the sample. Therefore, one unique fractionation will be enough to obtain the fractions amount needed for their complete characterization.
Experimental
To perform a fractionation, the user just needs to place a dry sample into a vessel, select the method and start the process. Once the fractionation is finished, the equipment is rinsed and ready to start a new one. The main steps of fractionation are performed automatically following the selected method parameters:
1. The vessel is filled with solvent and heated up for dissolution with stirring at high-temperature.
2. Column loading with the sample dissolved at high-temperature.
3. Crystallization step following the selected temperature ramp (when subambient conditions are required, PREP C20
can work without the need of liquid coolants down to minus 20ºC).
4. Elution step and fractions collection by pumping solvent at selected increasing isothermal steps (composition mode) or selected non-solvent percentage at a fixed temperature (molar mass mode). For TREF mode, the concentration of the different fractions that are coming out of the column is monitored with an infrared detector, which facilitates the
fractionation comprehension, so the analyst can modify the fractionation methods (elution volumes) according to his needs.
The control program allows the user to define different methods according to their needs. Therefore, dissolution temperatures or times, crystallization rates, elution volumes, etc. can be adjusted for each kind or amount of sample.
Fractionation according to composition (TREF mode) Chemical composition fractionation, in semicrystalline polymers, is based on differences in crystallizability of the polymer composition, and it is typically performed by crystallization at a slow cooling rate. Fractionation occurs by deposition of layers of decreasing crystallinity or increasing branch content, onto an inert support in the column, as temperature decreases.
Although at this stage the polymer is already segregated in layers or crystalline structures of different composition, the technique still requires a second temperature cycle to collect those fractions. This is achieved by pumping solvent meanwhile the temperature is being increased (elution step). The eluent dissolves fractions of increasing crystallinity, or decreasing branch content, as temperature rises. Figure 1 shows the fractionation of a complex dual reactor LLDPE polyolefin that was not possible to fractionate properly without using a support.
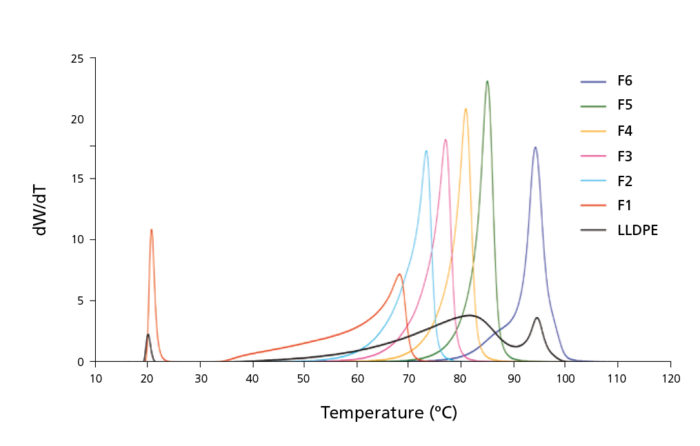
Figure 1. TREF curves of fractions obtained with PREP C20
and their LLDPE parent sample.
Fractionation according to molar mass
Molar mass fractionation has been implemented in PREP C20 using a solvent-non solvent gradient through the column at a constant temperature. Narrow fractions of increasing molar mass can be obtained as shown in Figure 2. The graph shows the molar mass distribution of a LLDPE and its fractions obtained with molar mass fractionation mode.
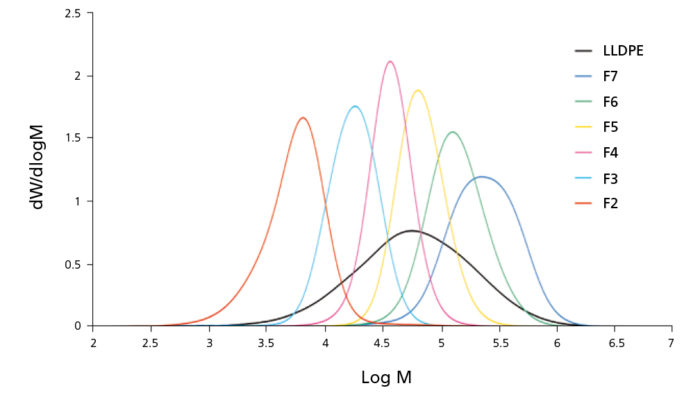
Figure 2. Molar Mass distribution of fractions obtained with PREP C20 and their LLDPE parent sample
Future and other perspectives
Developed initially for polyolefins, PREP C20 can also be used for other polymers depending on the solvents required for dissolution.
Currently, fractionation takes place according to TREF and molar mass modes, but other fractionation techniques such as thermal gradient interaction chromatography (TGIC) and solvent gradient interaction chromatography (SGIC) will be implemented soon.
Conclusions
Fractionation of polyolefins has always been a difficult and physically demanding task due to the manual manipulation of large volumes of hot solvent. After the first automation techniques, new complex polymers have shown to require a support to achieve a proper fractionation.
In this way, a column-based fractionation equipment has been developed, which also enables the fractionation of larger amounts of sample, in a completely automatic way.
The automated control program allows analysts to create their own methods adjusted to their samples and needs. The fractions are automatically collected in bottles, and it is possible to check the process performance thanks to an interactive software.
References
1. L. Wild and T. Ryle, Polym. Prepr. Am. Chem. Soc. Polym. Chem. Div., 18, 182 (1977).
2. T.E Davis and R.L. Tobias, Journal of Polymer Science. Vol 1, 227-243 (1961)
3. “Polymer Fractionation” F. Francuskiewicz. Edited by Springer (1994).
4. “Temperature rinsing elution fractionation and crystallization analysis fractionation” B. Monrabal
Encyclopedia of Analytical Chemistry. Edited by R.A. Meyers pp. 8074–8094. Wiley & Sons Ltd, (2000).







