September 2017
By B. Monrabal, T. González, P. del Hierro, A. Roig. Polymer Char, Valencia, Spain.
Introduction
Industrial polymer samples may contain fillers, gels/crosslinked material or are additivated with small-particle size carbon black as a light stabilizer. In those cases, a filtration step is required prior to a Liquid chromatography (LC) analysis to protect the life of the columns, and/or fouling the detector cells.
There are new instruments that may incorporate an in-line filter system prior to injection with a backflush mode, which can be effective for most standard samples contaminated with small amounts of residues (1). However, when samples contain a significant amount of crosslinked material or inorganic residues, an external filtration apparatus is advised, and it is a must when samples contain carbon black of very small-particle size due to the difficulties of removing it with standard filtration elements. Samples produced in batch reactors or pilot plants with reduced catalyst efficiency may also contain significant catalyst residues, which also need to be removed with an external filtration step.
The most difficult case is the removal of fine carbon black particles in polyolefins, usually analyzed by high-temperature GPC (Gel Permeation Chromatography), which is quite often used with a Light Scattering detector (LS). The presence of very small particles may damage frits or column packing, and the particles capable of passing through the column to the detector cell will result in a noisy baseline when using an LS detector. Thus, an efficient external filtration system is required in the analysis of these types of samples.
Instrumentation
An external filtration instrument capable of handling polymer solutions of up to 180ºC has been designed. It consists of two small modules:
1) An External Dissolution Oven (EDO) with a shaking motion for dissolution of polymer samples that require high temperature to dissolve. Samples are handled in vials of 10 and 20 mL volume and up to 6 samples can be dissolved simultaneously. Figure 1a.
2) An External Filtration System (EFS) with an oven and a vacuum system controlled by a microprocessor to filtrate one solution sample at a time. Figure 1b.
The filtration element is an aluminum cartridge capable of handling polymer solutions of up to 180ºC. It is filled with an inert, high surface packing and has top and bottom needles. The in-depth filtration cartridge has a clear advantage over a classical surface filtration element in terms of capacity and filtration speed, and it is the only alternative for completely removing fine particles such as carbon black.
The EFS oven has been designed to maintain at the desired temperature, the vial with the solution to be filtrated, the filtration cartridge, and the filtrated solution collecting vial. Figure 1c.
Filtration Procedure
- An empty vial, used to collect the filtered solution, is inserted in the bottom part of the EFS oven. Figure 1c.
- A new filtration cartridge is placed at the center of the EFS oven and the bottom needle pierces the empty collecting vial.
- The vial containing the polymer sample solution (typically with a concentration of 1 or 2 mg/mL) is taken out of the EDO unit at the dissolution temperature, placed in the top part of the EFS oven and then pierced by the top needle of the filtration cartridge element, Figure 1b and 1c.
- The start button initiates a pre-established filtration method. The aluminum cartridge is preheated in less than one minute and the vacuum pump is immediately turned ON to bring the initial polymer solution through the cartridge, and into the empty collecting vial, Figure 1c. Typically, a 20 mL vial is used to dissolve the sample in 10 mL of solvent. Roughly 8mL of the filtered solution are collected in a 10 mL vial. The remaining 2mL are kept at the bottom of the dissolution vial.
The whole process takes less than three minutes and the vial with the filtered solution can then be taken to the analytical equipment (GPC, HPLC, TREF…) for subsequent analysis.
The design of the filtration process prevents any possible cross-contamination because the polymer solution goes from the initial dissolution vial, through a single-use cartridge, and into the collecting vial, as shown in Figure 1c. The dissolution vial, now almost empty, and the used cartridge are disposed of properly, with no instrument cleaning required.
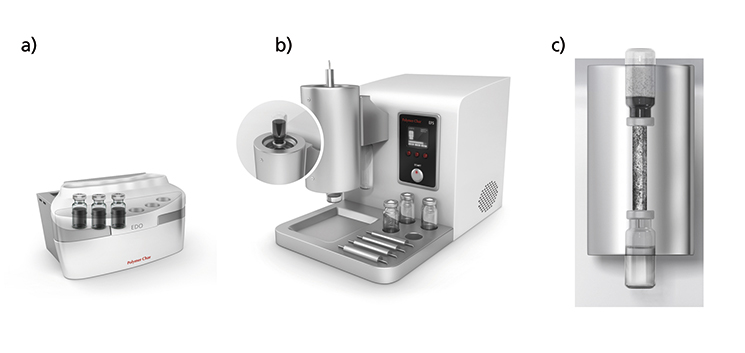
Figure 1. a) Schematic diagram of the external dissolution oven (EDO). b) View of the external filtration system (EFS). c) Schematic diagram of the three elements in contact with the polymer solution inside the EFS oven.
Results
The filtration process of the most difficult samples can be performed in a short time (less than three minutes). Figure 2a shows the initial solution of a Polyolefin containing 40% of carbon black and then the transparent solution obtained after going through the filtration cartridge, with the polymer precipitated to the upper layer when cooled down. Figure 2b shows the vial with the remaining solution volume and the used cartridge for disposal. All types of inorganic particles and gels are easily removed in a short time.
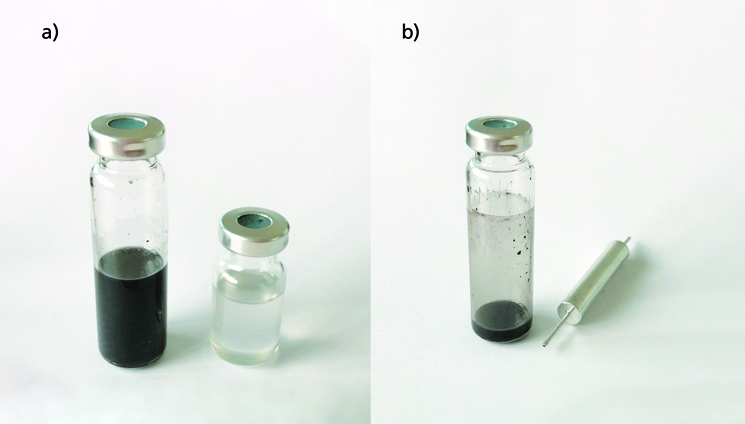
Figure 2. a) Polymer solution before and after EFS filtration. b) Disposable vial and cartridge after filtration.
Two different polyolefins were chosen to investigate if part of the polymer would be retained in the inert packing of the filter cartridge. For that purpose, the two samples were analyzed by GPC and the results of the original samples were compared to those after the solutions were filtered in the EFS.
Calculated recoveries of the samples filtered are close to 100%, indicating that no irreversible adsorption in the filter packing is taking place.
A linear low-density polyethylene (LLDPE) of MI 1 and a density of 0.920, was analyzed by GPC in triplicate, with and without filtration. The Molar mass distributions obtained show a perfect match as seen in Figure 3, indicating that neither degradation nor selective fractionation of this polymer happened in the filtration process.
The level of antioxidant (BHT) in the original solution remained constant after going through the filtration process; thus, ensuring that the same antioxidant level remains in the final analytical process.
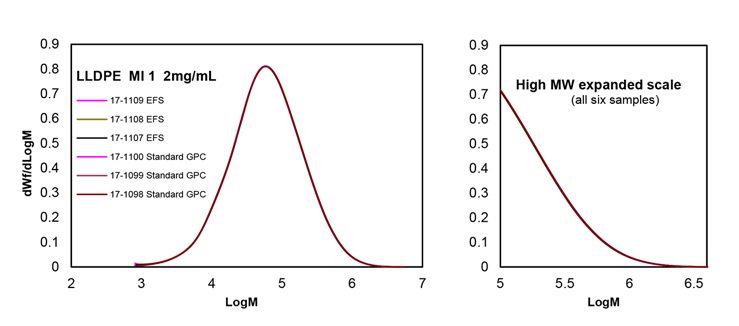
Figure 3. GPC analysis of an LLDPE sample by triplicate with and without EFS filtration.
An isotactic homopolymer polypropylene sample of 250000 molar mass was analyzed by GPC (triplicate analysis), with and without EFS filtration, as in the previous case but at a concentration of 1 mg/mL. The sample preparation incorporated a vial nitrogen purge to prevent thermal degradation in the dissolution step (2).
The molar mass distributions obtained are presented in Figure 4 and indicate that EFS filtration does not modify significantly the microstructure of the polypropylene samples (3).
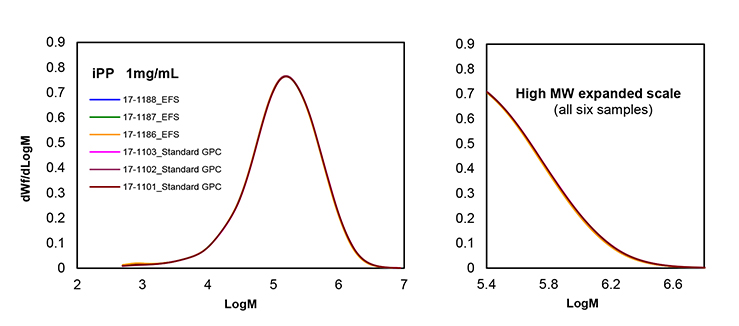
Figure 4. GPC analysis of a polypropylene sample by triplicate with and without EFS filtration.
Conclusion
A fast and effective filtration apparatus has been developed for Polymer solutions. It is prepared to handle polymers with large content of solids that demand high dissolution temperatures.
The filtration process is automated and proceeds in a closed environment in less than three minutes. The user does not require to handle solvents and the single-use cartridge design prevents any possible cross-contamination between samples.
The most difficult samples, such as polyolefins with a high content of carbon black, are easily filtered, resulting in a transparent solution.
No selective precipitation or fractionation of the sample takes place and the dissolved additives like BHT are not removed in the filtration process.
References
- Monrabal and W. Yau in Engineering advances in High-Temperature GPC Instrumentation, The Column 2011, LC/GC
- Monrabal in Polyolefin Characterization: Recent Advances in Separation Techniques, Vol. 257 (Ed. W. Kaminsky), Springer Berlin Heidelberg, 2013, pp. 203-251.
- González and B. Monrabal to be presented at ICPC 2018, Houston




